Organelle homeostasis
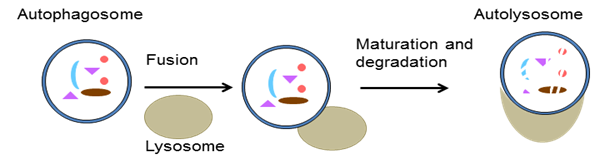
Autophagy is a membrane trafficking process by which endogenous or exogenous material is delivered to lysosomes, the primary catabolic compartments of eukaryotic cells. In this process, double-membrane vesicles (referred to as autophagosomes), assembled de novo in a series of steps, sequester cytosol containing intracellular proteins, damaged organelles, and exogenous material like microbes and fuse with lysosomes to form autophagolysosomes where the cargo is eventually degraded.
Autophagy was originally believed to be a catabolic process that helps cells survive under starvation conditions by restoring nutrient and energy balance. Subsequent findings however established a role for autophagy in various cellular processes involved in cell death, aging, metabolism, and host defense. Increasing evidence implicated autophagy with the pathogenesis of a wide variety of diseases, including certain myopathies, neurodegenerative diseases, and chronic infections.
We use different biochemical and imaging tools to understand how different organelle respond to various pathological conditions and study their Spatio-temporal regulation which will broaden our understanding of how the cell, as a large multi-subunit complex, shows the coordinated response to various stimuli.
Additionally, our research involves understanding cross-talk between cell death regulators such as caspases, inhibitors of apoptosis proteins (IAPs), and cell survival mechanisms such as NF-kappaB signaling pathways.
E3 ligases, DUBs and ubiquitin signalling
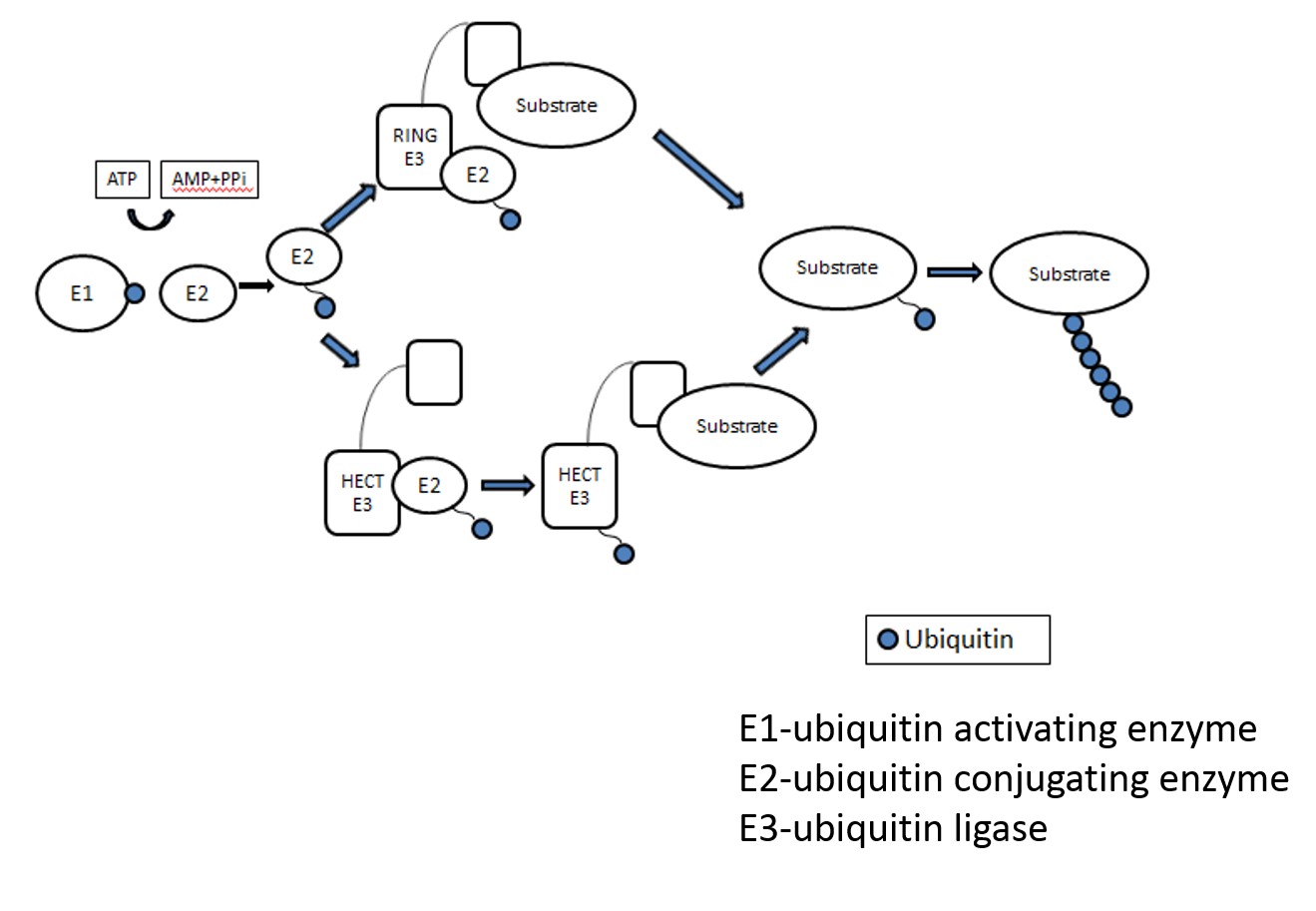
Ubiquitin modification is one of the most versatile post-translational modifications implicated in diverse cellular processes, including protein turnover, NF-κB signaling, cell death, protein and organelle trafficking, DNA repair, and inflammation. Understanding this dynamic modification is also necessary for developing novel therapeutic interventions against various human diseases linked to ubiquitination including cancer, immune disorders, and neurodegenerative diseases.
We are working on deciphering the role of novel E3 ligases & DUBs in the different cellular processes including autophagy/mitophagy, organelle dynamics, and ubiquitin signaling including linear ubiquitination (M1 linkage)
Host-pathogen interaction
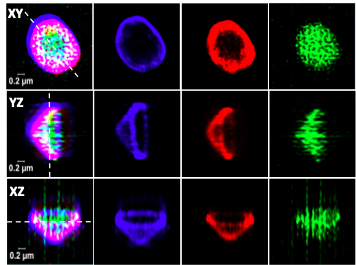
In response to infection, immune cells like macrophages and dendritic cells assemble ALIS [aggreosome like induced structures]. ALIS is known to be assembled by p62 and consists of ubiquitinated proteins and LC3. The physiological role of ALIS is not entirely clear. Structural analysis using SIM revealed that they are organized structures.
ALIS helps cells to cope with stress caused by infection and these structures are eliminated by autophagosomal machinery. We are interested in identifying the constituent that makes up ALIS and investigates the role played by ALIS in host defense.